How Many Cmv Babies Are Missed With a Hearing Screen
Introduction
Maternal primary and non-primary infection (exogenous reinfection with a different strain or endogenous viral reactivation) of cytomegalovirus (CMV) during pregnancy tin can upshot in in utero manual to the fetus (1). Infants can exist categorized every bit symptomatic or asymptomatic based on clinical symptoms/signs (Table 1) (2). Approximately 11% of alive-born infants born with congenital CMV (cCMV) have aberrant clinical findings at nascency (symptomatic) (three). Infants can experience substantial morbidity, mortality, and long-term sequelae, including sensorineural hearing loss (SNHL), the most common sequela (4, 5). Infants without symptoms at birth are also reported to be at hazard of developing long-term hearing loss (6). As a leading cause of congenital infections worldwide (7), cCMV infection meets many of the criteria for screening: it is clinically of import, well defined and prevalent (4). Nevertheless, neither universal antenatal screening for CMV during pregnancy nor universal neonatal screening is routinely recommended (eight) and there remain several challenges that impede their implementation. Roche Centralised and Betoken of Intendance Solutions and Roche Molecular Diagnostics convened a group of CMV experts (microbiologists, virologists, and clinicians) to discuss and offer strategies to address these barriers and knowledge gaps. This paper provides an overview of those discussions and is a narrative review of serologic and viral nucleic acid screening and diagnostics in the context of maternal, fetal and neonatal CMV infection.
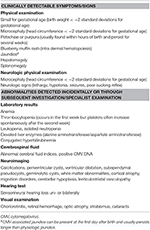
Table i. Possible signs and symptoms in children with congenital CMV (reproduced from Luck et al.).
Maternal CMV Screening
CMV screening is offered to some pregnant women in parts of Europe, Israel, Australia and the USA in the setting of population-based studies, and independently of nationally endorsed screening programs (9). However, universal antenatal screening for CMV is not routinely recommended (8). Reasons non to screen include the absenteeism of medication to prevent transmission and the difficulty of predicting sequelae (10).
The introduction of routine testing for CMV in pregnant women has several implications. Despite the difficulties mentioned higher up, the virtually important benefit of screening would be to identify fetuses at hazard of developing sequelae.
Maternal screening, ideally early on in the start trimester, would as well identify those who were CMV-seronegative and thus allow information to exist provided regarding hygiene and behavioral measures to prevent CMV infection. Prove has shown that intervention based on the identification and hygiene counseling of CMV-seronegative pregnant women significantly prevents maternal infection (xi). Hygiene counseling may likewise provide (equally yet unproven) benefits for those who are seropositive.
During early pregnancy, repeat serologic screening with CMV-specific immunoglobulin G (IgG) and -M (IgM) antibodies of previously seronegative pregnant women at the terminate of the outset trimester (or until week xx) would place maternal primary CMV infection. Although in that location are no universally accepted guidelines, testing earlier 18–20 weeks of pregnancy is reasonable in order to place belatedly seroconversion at the terminate of the first trimester and implement fetal investigations. In the event of seroconversion, parents should exist informed of the chance of vertical transmission [32% (3)] and the possible consequences.
Other strategies that could be put in place include maternal screening at the beginning prenatal visit and at birth, and neonatal screening at birth for those whose mothers tested seropositive, with diagnosis of the neonate by saliva or urine CMV Dna detection. Previously, this approach to targeted testing detected 82% of all cCMV infections (12). Notably, this written report utilized culture for diagnosis; the detection rate is probable to be improved with polymerase chain reaction (PCR). This approach may be more cost-effective than screening all children.
However, screening tests do not place which mothers volition transmit the virus (13). Moreover, in that location are no surrogate markers to predict whether infection in the infant will lead to long-term sequelae.
Lastly, there are also risks for the mother associated with maternal screening for CMV that include the stress of having extra tests, the potential for unnecessary terminations (14), the potential risk of miscarriage or stillbirth from confirmatory amniocentesis [inversely correlated to skill/experience of operator (xv)], and the cost. The challenge is in providing women with choice and information in the context of population-based economics.
Serologic and Molecular Testing for Maternal CMV Infection
Chief infections can be identified by serologic testing. During pregnancy, IgG and IgM serology is the preferred option; IgG ardor testing should be used just if CMV-specific IgM antibodies are positive. Many laboratories consider IgM-positive results, in combination with IgG avidity results, to discriminate between main and not-primary CMV infections (16). Low CMV IgG avidity indicates primary infection within the preceding 3–4 months, with an increased risk of intrauterine manual to the fetus (17).
CMV-specific serology testing is nearly useful in the first trimester due to the increased severity of illness when primary infection occurs during the embryonic or early fetal flow (18). Quantitative IgG testing is helpful to find seroconversion and the stage of infection. Low levels of CMV-IgG antibodies in maternal serum samples nowadays challenges for the clinician: depression IgG levels can be associated with both a truthful positive or a simulated-positive consequence (19), so clear guidelines are needed for the appropriate interpretation of serology results.
All serologic kits vary considerably in their accuracy of the "low" range of IgG values; a very low value in i test may be negative using a different test. CMV-IgG avidity testing should not exist performed on serum samples with low IgG levels as these tin can give inappropriate IgG avidity results (20). An wrong classification of chief CMV infection tin can pb to inappropriate management. Finally, maternal serology screening can be falsely reassuring equally non-primary maternal infections volition non be recognized: in Europe, this represents effectually fifty% of all cCMV cases (21, 22).
The new WHO standard (23) was established for the scale of anti-CMV IgG diagnostic kits with quantitative test interpretation and as an assist in the interpretation of serologic results in the framework of different assays, platforms, and clinical settings. The CMV standard could be of value, although in other settings such every bit rubella or toxoplasmosis screening, the use of a standard for calibrating IgG assays has proven to be suboptimal (24, 25). An algorithm for dealing with depression positive IgG samples may be more useful than a WHO standard. In particular, in the absence of a gold standard method, an equivocal IgG serologic assay event in a pregnant adult female should be considered negative. This strategy will ensure that these women are assigned to the highest CMV hazard group for pregnancy outcome.
The value of viral DNA detection and quantification in blood, saliva, or urine to assist determine the timing of maternal infection, or to gauge the risk of fetal transmission, is non withal established. Notably, two studies accept demonstrated that persisting levels of maternal DNAemia during primary CMV infection at the moment of amniocentesis correlate with a loftier risk of CMV transmission to the fetus (26, 27), whilst ane other report has shown that the presence of CMV Deoxyribonucleic acid in maternal urine and maternal blood correlated with transmission of CMV to offspring (28).
Prenatal Diagnosis of Fetal CMV Infection
Ultrasound imaging has poor sensitivity in diagnosing fetal CMV infection (29) but is a useful tool to predict the prognosis of fetal infection. Diagnosis of fetal CMV infection by CMV PCR in the amniotic fluid can be fabricated with high sensitivity and specificity by amniocentesis later on 20–21 weeks' gestation (30) [and >8 weeks after estimated maternal seroconversion (31)] and is the best available prenatal diagnostic tool (32). When the diagnosis of fetal infection is by way of amniocentesis, the prognostic evaluation of fetal infection relies on imaging using a combination of ultrasound and cognitive magnetic resonance imaging (MRI). Several studies take identified a residual risk of hearing loss at nativity when imaging (ultrasound and/or MRI) test was considered to exist normal (33–38).
Neonatal CMV Screening
Neonatal CMV screening would enable early detection of cCMV (following principal and not-principal maternal infection), simply universal neonatal screening for CMV is currently not recommended by any public health body. Data from Uematsu and colleagues emphasize that without neonatal screening some infected neonates that develop neurological sequelae may go unrecognized (39).
Although universal screening is not performed, targeted screening of newborns who fail the neonatal hearing test has been implemented in some hospitals and states in the U.s.a. (40, 41). In the UK, Belgium and Australia, targeted testing of infants who were referred for farther audiological testing (after failing the routine hearing screening) has too been trialed with some success (42–44). This combination of targeted newborn screening and early detection and interventions is probable to benefit children with cCMV (45).
Additionally, the costs associated with targeted neonatal screening look favorable compared with other screening programs (46, 47). Withal, this targeted approach would miss those CMV-positive infants who pass the newborn hearing test but are still at risk for late-onset SNHL (40, 48). In i study, 43% of infants with CMV-related SNHL in the neonatal period and cCMV infants who are at hazard for late-onset SNHL were not identified by newborn hearing screening (49).
There are risks associated with neonatal screening, such as the potential for parental anxiety while waiting for confirmatory testing results. In addition, at that place may exist feet related to the extended flow of audiological monitoring that a cCMV-positive infant must undergo [upwards to 6 years (2, fifty, 51)]. Most cCMV infections are asymptomatic and do not present a risk for the onset of belatedly sequelae (five). Recent information demonstrated that primary maternal infections before the 14th week of pregnancy, the presence of a disseminated infection at nascency, and imaging abnormalities in the neonate were adventure factors for SNHL (52). This is a step toward the development of neonatal predictive markers that can be used to place those at high run a risk of developing sequelae.
Diagnosis and Screening of Neonatal Infection
Testing is recommended for those who have any condition that might exist indicative of intrauterine CMV infection (2). Traditionally, viral isolation and culture from urine or saliva was the standard for diagnosing cCMV infection (53). Since PCR exhibits high sensitivity (54, 55), this is now the preferred option. False-positive tests have been reported for saliva, and therefore any positive saliva result should be confirmed by CMV detection in urine (21).
It should be noted that if the diagnosis is made later the first ii–3 weeks of life (2, 9), infection may have been postnatally acquired and attributable to infected breast milk from a seropositive mother (56), rather than cCMV. In this instance, congenital infection must exist confirmed by detection of CMV from a sample taken at nativity.
Saliva tin can exist used to screen for cCMV; notwithstanding, equally this specimen type is not routinely collected from neonates, a change in infrastructure would be required before this could be rolled out on a large calibration. Equally such, alternative technologies for universal screening are currently under evaluation. Due to widespread utilization in neonatal screening for other conditions, there has been much interest in using dried blood spots (DBS) taken at nativity for CMV screening. However, screening DBS is less sensitive than PCR testing of saliva, with sensitivity ranging between 28 and 100% (57), and is contingent upon the method of extraction and Deoxyribonucleic acid amplification and the patient group selected. The recent standardization of viral DNA extraction and innovative PCR techniques has led to improved sensitivity of DBS screening to around 80% (58). A potential limitation in the apply of DBS is that simply fourscore–90% of congenitally infected infants have detectable CMV in their blood shortly afterwards birth (59, 60). Despite this, the sensitivity of DBS screening has been shown to adequately detect those most at risk of developing SNHL (61).
Stored DBS tin exist used to diagnose cCMV retrospectively (2). In some countries (e.g., Frg), the use of DBS for retrospective diagnosis or screening of newborns is hampered past the destruction of samples after iii months (62) due to information protection requirements. Thus, for sure countries, regulatory changes may be necessary to permit long-term storage and use in this context.
What are the Gaps in our Understanding?
Although pregnant advancements accept been fabricated, many questions remain unanswered in this field (Table 2). Well-designed clinical trials to address several facets of CMV treatment (in significant women, CMV-infected fetuses and both symptomatic and asymptomatic neonates and children) are required. Prevention (vaccines), biology, and transmission factors associated with not-chief CMV, and the cost-effectiveness of universal screening, all need further exploration to fully realize the ultimate goal of preventing cCMV.
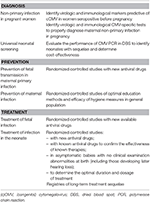
Tabular array 2. Studies required to better the understanding of congenital CMV.
Currently, treatment with immunoglobulins or antiviral therapy to prevent intrauterine manual of CMV in pregnant women with main CMV infection is not recommended as studies take not yet conclusively shown a benefit (63–66). Data from a non-randomized study showed that biweekly administration of hyperimmunoglobulin until 20 weeks' gestation successfully prevented maternal-fetal transmission of main infections (65). These data need to exist confirmed past a randomized clinical trial (67); if the results are confirmed, ii-weekly intervals for testing seronegative women would be necessary. Information technology has been suggested that a study that demonstrates treatment efficacy resulting in at least a 47% reduction in cCMV disease would brand universal screening and treating for principal CMV in pregnancy cost-effective (10).
Whilst recent improvements in screening and diagnosis permit detection of main CMV infection in pregnancy, unfortunately treatment options for CMV-infected fetuses and neonates are express due to bereft evidence for safety and effectiveness. Consequently, routine antiviral therapy to treat fetal CMV during pregnancy is not recommended (nine). In infants with clinical disease at nascence, early intervention with ganciclovir or valganciclovir can prevent hearing deterioration and improve developmental outcomes, although both treatments are associated with neutropenia (68, 69) and other possible long-term effects (lxx). Currently, valganciclovir handling is recommended based on severity or number of symptoms. A European expert consensus statement recommends that treatment with oral valganciclovir (intravenous ganciclovir under certain circumstances) is just for those with: bear witness of central nervous system disease; evidence of life-threatening illness, astringent single-organ disease or multi-organ involvement; "moderate" cCMV illness once discussed on a case-past-case basis with a clinician with feel of managing infants with cCMV (2). The informal International Congenital Cytomegalovirus Recommendations Grouping recommend oral valganciclovir treatment for those neonates with "moderately" to "severely" symptomatic cCMV illness (9). Further development of efficacious antivirals with an adequate safety contour is required.
Letermovir is a new amanuensis approved for utilise in the prophylaxis of CMV infection in CMV-seropositive recipients of an allogeneic hematopoietic stem cell transplant over the historic period of 18 years (71). A recent case study revealed potential efficacy in pediatric allogeneic hematopoietic stem cell transplant patients (72). Further studies are required to make up one's mind whether information technology is safe and effective for treating those with cCMV.
More asymptomatic infants will be discovered if screening programs become widespread. Currently, antiviral therapy for asymptomatic infants is not recommended (2) but, like symptomatic infants, they are at risk of developing belatedly-onset sequelae (53). Trials that investigate which interventions are effective and prophylactic for asymptomatic infants or those with isolated hearing loss or subtle neuroimaging abnormalities, equally well as older children that develop late-onset hearing loss, are necessary. Since clinical trials are ongoing (73–75), some of these points will hopefully be clarified in the next few years and the best direction plan for this population determined.
As well as trials targeting treatment for specific populations, data on prevention are also required. It is known that natural infection confers some protection confronting both horizontal and vertical transmission (13, 76), therefore the development of an effective vaccine is feasible. Clinical trials of CMV vaccines should evaluate protection against cCMV infection (77). Recent modeling information of a single fictional cohort of 390,000 adolescent women propose that vaccination could be cost-effective (78).
In the absence of a vaccine to forbid infection, a greater focus on education and prevention strategies for cCMV infection are needed for women intending to become significant, those already pregnant, and healthcare professionals alike. Preconception screening in those attending a fertility clinic, with resultant counseling to improve personal hygiene in those who were not immune to CMV, has shown that hygiene counseling (albeit in this highly selected accomplice) is effective in reducing CMV exposure (79). In meaning women, hygiene counseling of CMV-seronegative pregnant women significantly prevents maternal infection (11). De Vries and colleagues showed that non-chief infections business relationship for the majority of CMV-related hearing loss, suggesting prevention enquiry should encompass all significant women, non but those who are seronegative (80). Therefore, the current method aimed at preventing transmission of CMV—education concerning hygiene measures to be taken around pocket-sized children [such equally avoiding kissing babies on the mouth, not sharing cutlery with immature children, and paw hygiene after a diaper modify (11)]—should be performed regardless of knowing the female parent'due south serostatus. However, in the full general population, there is inadequate bear witness to show that education translates into a decrease in maternal infection (iv). Studies assessing the efficacy of hygiene measures on the prevention of CMV infection in pregnancy, and resultant cCMV, are necessary in women of reproductive age and are ongoing in the UK (81).
Clinicians practice not know whether a not-primary infection is a reactivation or an infection with another strain of CMV. This distinction may be of import in understanding the etiology of CMV illness, thus more enquiry on the office of non-primary maternal CMV infections in congenital infection is necessary. At nowadays, there are no tools validated to place women at gamble of transmitting the virus afterwards a non-main infection.
Finally, further evidence of cost-effectiveness is required. Whilst the cost-effectiveness of universal and targeted newborn cCMV screening programs has been assessed in the United kingdom and USA (46, 47), and the economical burden of cCMV in the Uk estimated (82), currently there are insufficient price-do good data, which hinders the implementation of screening.
Conclusions
cCMV infection results in significant consequences for the infected neonate. Despite this, universal maternal or neonatal screening for CMV and cCMV is not routinely recommended. A summary of our electric current recommendations for diagnosis, screening, and prevention is provided in Table iii. Presently there are pregnant gaps in understanding that prevent the implementation of universal screening, including insufficient data on price-effectiveness and the lack of evidence for rubber and efficacious treatments for those infected. Additionally, further data on non-primary maternal infection and the risk of cCMV infection are necessary. In the virtually future, we are confident that many aspects related to diagnosis, maternal and fetal therapy, and active prevention will surely nowadays an improvement and our recommendations may change. Until then, and in the absence of a vaccine, hygiene recommendations to prevent CMV infection should exist made to all pregnant women.
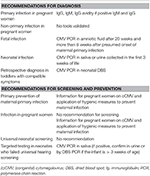
Tabular array 3. Expert panel recommendations for the diagnosis, screening, and prevention of CMV infection.
Author Contributions
TL and ML-Five wrote the starting time typhoon of the manuscript. All authors (TL, DB-G, Yard-LD, IF, SL, SM, and ML-V) contributed to manuscript revision, read and approved the submitted version.
Funding
This work was supported by Roche Diagnostics Ltd.
Conflict of Interest
TL, G-LD, IF, SL, and SM report personal fees from Roche Diagnostics Ltd. DB-G reports personal fees from Roche Diagnostics Ltd. and MSD. ML-Five reports personal fees from Roche Diagnostics Ltd., not-financial and other support from BioMérieux, non-financial support from Abbott, and not-financial support from Ferring SAS.
Acknowledgments
Editorial aid was provided by Corrinne Segal, Ph.D., of Elements Communications Ltd., Westerham, Great britain.
Abbreviations
cCMV, Congenital cytomegalovirus; CMV, Cytomegalovirus; DBS, stale blood spots; IgG, Immunoglobulin G; IgM, Immunoglobulin Thousand; MRI, magnetic resonance imaging; PCR, polymerase concatenation reaction; SNHL, Sensorineural hearing loss.
References
i. Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol. (2010) 20:202–13. doi: 10.1002/rmv.655
PubMed Abstruse | CrossRef Total Text | Google Scholar
2. Luck SE, Wieringa JW, Blázquez-Gamero D, Henneke P, Schuster K, Butler K, et al. Congenital cytomegalovirus: a European expert consensus argument on diagnosis and management. Pediatr Infect Dis J. (2017) 36:1205–13. doi: ten.1097/INF.0000000000001763
PubMed Abstract | CrossRef Full Text | Google Scholar
5. Goderis J, De Leenheer E, Smets Grand, Van Hoecke H, Keymeulen A, Dhooge I. Hearing loss and congenital CMV infection: a systematic review. Pediatrics. (2014) 134:972–82. doi: 10.1542/peds.2014-1173
PubMed Abstract | CrossRef Full Text | Google Scholar
6. Bartlett AW, McMullan B, Rawlinson WD, Palasanthiran P. Hearing and neurodevelopmental outcomes for children with asymptomatic built cytomegalovirus infection: a systematic review. Rev Med Virol. (2017) 27:e1938. doi: x.1002/rmv.1938
CrossRef Total Text | Google Scholar
7. Manicklal S, Emery VC, Lazzarotto T, Boppana SB, Gupta RK. The "silent" global burden of congenital cytomegalovirus. Clin Microbiol Rev. (2013) 26:86–102. doi: ten.1128/CMR.00062-12
PubMed Abstruse | CrossRef Full Text | Google Scholar
8. The American Higher of Obstetricians and Gynecologists. Do bulletin no. 151: cytomegalovirus, parvovirus B19, varicella zoster, and toxoplasmosis in pregnancy. Obstet Gynecol. (2015) 125:1510–25. doi: 10.1097/01.AOG.0000466430.19823.53
CrossRef Full Text | Google Scholar
9. Rawlinson WD, Boppana SB, Fowler KB, Kimberlin DW, Lazzarotto T, Alain S, et al. Congenital cytomegalovirus infection in pregnancy and the neonate: consensus recommendations for prevention, diagnosis, and therapy. Lancet Infect Dis. (2017) 17:e177–88. doi: ten.1016/S1473-3099(17)30143-3
PubMed Abstract | CrossRef Total Text | Google Scholar
10. Cahill AG, Odibo AO, Stamilio DM, Macones GA. Screening and treating for primary cytomegalovirus infection in pregnancy: where exercise we stand up? A determination-analytic and economic analysis. Am J Obstet Gynecol. (2009) 201:466.e1–7. doi: 10.1016/j.ajog.2009.07.056
PubMed Abstract | CrossRef Full Text | Google Scholar
11. Revello MG, Tibaldi C, Masuelli Thou, Frisina V, Sacchi A, Furione Chiliad, et al. Prevention of primary cytomegalovirus infection in pregnancy. EBioMed. (2015) 2:1205–10. doi: 10.1016/j.ebiom.2015.08.003
PubMed Abstract | CrossRef Full Text | Google Scholar
12. Naessens A, Casteels A, Decatte L, Foulon W. A serologic strategy for detecting neonates at risk for built cytomegalovirus infection. J Pediatr. (2005) 146:194–seven. doi: 10.1016/j.jpeds.2004.09.025
PubMed Abstruse | CrossRef Full Text | Google Scholar
13. Lilleri D, Gerna 1000. Maternal immune correlates of protection from human cytomegalovirus manual to the fetus after primary infection in pregnancy. Rev Med Virol. (2017) 27:e1921. doi: 10.1002/rmv.1921
PubMed Abstruse | CrossRef Full Text | Google Scholar
14. Guerra B, Simonazzi G, Banfi A, Lazzarotto T, Farina A, Lanari M, et al. Impact of diagnostic and confirmatory tests and prenatal counseling on the rate of pregnancy termination among women with positive cytomegalovirus immunoglobulin M antibiotic titers. Am J Obstet Gynecol. (2007) 196:221.e1–half-dozen. doi: 10.1016/j.ajog.2006.08.039
PubMed Abstruse | CrossRef Total Text | Google Scholar
15. Wulff CB, Gerds TA, Rode L, Ekelund CK, Petersen OB, Tabor A. Risk of fetal loss associated with invasive testing following combined commencement-trimester screening for Down syndrome: a national cohort of 147,987 singleton pregnancies. Ultrasound Obstet Gynecol. (2016) 47:38–44. doi: 10.1002/uog.15820
PubMed Abstract | CrossRef Total Text | Google Scholar
xvi. Prince HE, Lape-Nixon M. Function of cytomegalovirus (CMV) IgG avidity testing in diagnosing primary CMV infection during pregnancy. Clin Vaccine Immunol. (2014) 21:1377–84. doi: 10.1128/CVI.00487-14
PubMed Abstruse | CrossRef Full Text | Google Scholar
17. Lazzarotto T, Guerra B, Gabrielli L, Lanari 1000, Landini MP. Update on the prevention, diagnosis and management of cytomegalovirus infection during pregnancy. Clin Microbiol Infect. (2011) 17:1285–93. doi: 10.1111/j.1469-0691.2011.03564.x
PubMed Abstract | CrossRef Full Text | Google Scholar
18. Faure-Bardon V, Magny JF, Parodi Yard, Couderc S, Garcia P, Maillotte AM, et al. Sequelae of congenital cytomegalovirus following maternal primary infections are limited to those acquired in the first trimester of pregnancy. Clin Infect Dis. (2019) 69:1526–32. doi: 10.1093/cid/ciy1128
PubMed Abstruse | CrossRef Total Text | Google Scholar
19. Furione One thousand, Sarasini A, Arossa A, Fornara C, Lilleri D, Perez 50, et al. False human being cytomegalovirus IgG-positivity at prenatal screening. J Clin Virol. (2018) 104:34–8. doi: 10.1016/j.jcv.2018.04.009
PubMed Abstract | CrossRef Full Text | Google Scholar
20. Berth One thousand, Grangeot-Keros L, Heskia F, Dugua JM, Vauloup-Fellous C. Belittling issues possibly affecting the performance of commercial human cytomegalovirus IgG ardor assays. Eur J Clin Microbiol Infect Dis. (2014) 33:1579–84. doi: x.1007/s10096-014-2109-8
PubMed Abstract | CrossRef Full Text | Google Scholar
21. Leruez-Ville M, Magny JF, Couderc S, Pichon C, Parodi M, Bussieres 50, et al. Risk factors for congenital cytomegalovirus infection following primary and nonprimary maternal infection: a prospective neonatal screening study using polymerase chain reaction in saliva. Clin Infect Dis. (2017) 65:398–404. doi: x.1093/cid/cix337
PubMed Abstruse | CrossRef Total Text | Google Scholar
22. Puhakka Fifty, Lappalainen M, Lonnqvist T, Niemensivu R, Lindahl P, Nieminen T, et al. The burden of congenital cytomegalovirus infection: a prospective cohort study of twenty 000 infants in Finland. J Pediatric Infect Dis Soc. (2018) eight:205–12. doi: x.1093/jpids/piy027
PubMed Abstruse | CrossRef Full Text | Google Scholar
24. Maudry A, Chene G, Chatelain R, Patural H, Bellete B, Tisseur B, et al. Bicentric evaluation of half dozen anti-toxoplasma immunoglobulin Yard (IgG) automatic immunoassays and comparing to the Toxo II IgG Western blot. Clin Vaccine Immunol. (2009) sixteen:1322–6. doi: ten.1128/CVI.00128-09
PubMed Abstract | CrossRef Total Text | Google Scholar
26. Zavattoni M, Furione Grand, Lanzarini P, Arossa A, Rustico M, Tassis B, et al. Monitoring of human cytomegalovirus DNAemia during principal infection in transmitter and non-transmitter mothers. J Clin Virol. (2016) 82:89–93. doi: 10.1016/j.jcv.2016.07.005
PubMed Abstract | CrossRef Full Text | Google Scholar
27. Simonazzi Yard, Cervi F, Zavatta A, Pellizzoni L, Guerra B, Mastroroberto Thou, et al. Congenital cytomegalovirus infection: prognostic value of maternal DNAemia at amniocentesis. Clin Infect Dis. (2017) 64:207–10. doi: 10.1093/cid/ciw700
PubMed Abstruse | CrossRef Total Text | Google Scholar
28. Delforge ML, Costa East, Brancart F, Goldman D, Montesinos I, Zaytouni S, et al. Presence of cytomegalovirus in urine and blood of pregnant women with principal infection might exist associated with fetal infection. J Clin Virol. (2017) 90:14–17. doi: x.1016/j.jcv.2017.03.004
PubMed Abstract | CrossRef Full Text | Google Scholar
29. Guerra B, Simonazzi Thou, Puccetti C, Lanari M, Farina A, Lazzarotto T, et al. Ultrasound prediction of symptomatic congenital cytomegalovirus infection. Am J Obstet Gynecol. (2008) 198:380.e1–7. doi: x.1016/j.ajog.2007.09.052
PubMed Abstruse | CrossRef Full Text | Google Scholar
30. Liesnard C, Donner C, Brancart F, Gosselin F, Delforge ML, Rodesch F. Prenatal diagnosis of congenital cytomegalovirus infection: prospective study of 237 pregnancies at risk. Obstet Gynecol. (2000) 95(6 Pt 1):881–8. doi: 10.1097/00006250-200006000-00019
PubMed Abstract | CrossRef Full Text | Google Scholar
31. Enders One thousand, Daiminger A, Exler Southward, Ertan 1000, Enders Chiliad, Bald R. Prenatal diagnosis of congenital cytomegalovirus infection in 115 cases: a 5 years' single center experience. Prenat Diagn. (2017) 37:389–98. doi: ten.1002/pd.5025
PubMed Abstruse | CrossRef Full Text | Google Scholar
32. Society for Maternal-Fetal Medicine (SMFM), Hughes BL, Gyamfi-Bannerman C. Diagnosis and antenatal management of congenital cytomegalovirus infection. Am J Obstet Gynecol. (2016) 214:B5–eleven. doi: 10.1016/j.ajog.2016.02.042
PubMed Abstruse | CrossRef Full Text | Google Scholar
33. Farkas N, Hoffmann C, Ben-Sira L, Lev D, Schweiger A, Kidron D, et al. Does normal fetal encephalon ultrasound predict normal neurodevelopmental outcome in congenital cytomegalovirus infection? Prenat Diagn. (2011) 31:360–6. doi: x.1002/pd.2694
PubMed Abstract | CrossRef Full Text | Google Scholar
34. Faure-Bardon V, Millischer AE, Deloison B, Sonigo P, Grévent D, Salomon 50, et al. Refining the prognosis of fetuses infected with cytomegalovirus in the starting time trimester of pregnancy by series prenatal assessment: a unmarried-eye retrospective study. BJOG. (2020) 127:355–62. doi: 10.1111/1471-0528.15935
PubMed Abstract | CrossRef Total Text | Google Scholar
35. Picone O, Simon I, Benachi A, Brunelle F, Sonigo P. Comparison betwixt ultrasound and magnetic resonance imaging in assessment of fetal cytomegalovirus infection. Prenat Diagn. (2008) 28:753–8. doi: ten.1002/pd.2037
PubMed Abstract | CrossRef Full Text | Google Scholar
36. Lipitz Due south, Yinon Y, Malinger G, Yagel S, Levit L, Hoffman C, et al. Risk of cytomegalovirus-associated sequelae in relation to fourth dimension of infection and findings on prenatal imaging. Ultrasound Obstet Gynecol. (2013) 41:508–fourteen. doi: 10.1002/uog.12377
PubMed Abstract | CrossRef Full Text | Google Scholar
37. Leruez-Ville Thousand, Stirnemann J, Sellier Y, Guilleminot T, Dejean A, Magny JF, et al. Feasibility of predicting the outcome of fetal infection with cytomegalovirus at the time of prenatal diagnosis. Am J Obstet Gynecol. (2016) 215:342.e1–ix. doi: 10.1016/j.ajog.2016.03.052
PubMed Abstract | CrossRef Full Text | Google Scholar
38. Enders G, Bader U, Lindemann L, Schalasta One thousand, Daiminger A. Prenatal diagnosis of congenital cytomegalovirus infection in 189 pregnancies with known outcome. Prenat Diagn. (2001) 21:362–77. doi: 10.1002/pd.59
PubMed Abstract | CrossRef Total Text | Google Scholar
39. Uematsu M, Haginoya K, Kikuchi A, Hino-Fukuyo Northward, Ishii K, Shiihara T, et al. Asymptomatic congenital cytomegalovirus infection with neurological sequelae: a retrospective study using umbilical string. Brain Dev. (2016) 38:819–26. doi: x.1016/j.braindev.2016.03.006
PubMed Abstract | CrossRef Full Text | Google Scholar
40. Vancor E, Shapiro ED, Loyal J. Results of a targeted screening program for congenital cytomegalovirus infection in infants who fail newborn hearing screening. J Pediatric Infect Dis Soc. (2019) eight:55–9. doi: 10.1093/jpids/pix105
PubMed Abstract | CrossRef Full Text | Google Scholar
41. Diener ML, Zick CD, McVicar SB, Boettger J, Park AH. Outcomes from a hearing-targeted cytomegalovirus screening program. Pediatrics. (2017) 139:e20160789. doi: 10.1542/peds.2016-0789
PubMed Abstract | CrossRef Full Text | Google Scholar
42. Williams EJ, Kadambari S, Berrington JE, Luck S, Atkinson C, Walter S, et al. Feasibility and acceptability of targeted screening for congenital CMV-related hearing loss. Curvation Dis Kid Fetal Neonatal Ed. (2014) 99:F230–half dozen. doi: 10.1136/archdischild-2013-305276
PubMed Abstract | CrossRef Total Text | Google Scholar
43. Beswick R, David G, Higashi H, Thomas D, Nourse C, Koh Grand, et al. Integration of congenital cytomegalovirus screening within a newborn hearing screening programme. J Paediatr Child Health. (2019) 55:1381–8. doi: 10.1111/jpc.14428
PubMed Abstract | CrossRef Full Text | Google Scholar
44. Courtmans I, Mancilla Five, Ligny C, Le Bon SD, Naessens A, Foulon I. Incidence of congenital CMV in children at a hearing rehabilitation eye. B-ENT. (2015) xi:303–8.
PubMed Abstract | Google Scholar
45. Cannon MJ, Griffiths PD, Aston V, Rawlinson WD. Universal newborn screening for built CMV infection: what is the evidence of potential benefit? Rev Med Virol. (2014) 24:291–307. doi: x.1002/rmv.1790
PubMed Abstruse | CrossRef Total Text | Google Scholar
46. Williams EJ, Greyness J, Luck S, Atkinson C, Embleton ND, Kadambari S, et al. First estimates of the potential cost and toll saving of protecting childhood hearing from damage caused past congenital CMV infection. Arch Dis Kid Fetal Neonatal Ed. (2015) 100:F501–6. doi: ten.1136/archdischild-2014-306756
PubMed Abstract | CrossRef Full Text | Google Scholar
47. Gantt S, Dionne F, Kozak FK, Goshen O, Goldfarb DM, Park AH, et al. Cost-effectiveness of universal and targeted newborn screening for congenital cytomegalovirus infection. JAMA Pediatr. (2016) 170:1173–80. doi: x.1001/jamapediatrics.2016.2016
PubMed Abstract | CrossRef Total Text | Google Scholar
48. Fowler One thousand. Targeted CMV screening and hearing management of children with built cytomegalovirus infection. ENT Audiol News. (2018) 27.
Google Scholar
49. Fowler KB, McCollister FP, Sabo DL, Shoup AG, Owen KE, Woodruff JL, et al. A targeted approach for built cytomegalovirus screening within newborn hearing screening. Pediatrics. (2017) 139:e20162128. doi: 10.1542/peds.2016-2128
PubMed Abstract | CrossRef Full Text | Google Scholar
50. Lanzieri TM, Chung W, Flores Grand, Blum P, Caviness AC, Bialek SR, et al. Hearing loss in children with asymptomatic congenital cytomegalovirus infection. Pediatrics. (2017) 139:e20162610. doi: 10.1542/peds.2016-2610
PubMed Abstract | CrossRef Full Text | Google Scholar
51. Foulon I, Vleurinck L, Kerkhofs K, Gordts F. Hearing configuration in children with cCMV infection and proposal of a flow chart for hearing evaluation. Int J Audiol. (2015) 54:714–ix. doi: 10.3109/14992027.2015.1046506
PubMed Abstruse | CrossRef Full Text | Google Scholar
52. Foulon I, De Brucker Y, Buyl R, Lichtert E, Verbruggen K, Pierard D, et al. Hearing loss with congenital cytomegalovirus infection. Pediatrics. (2019) 144:e20183095. doi: 10.1542/peds.2018-3095
PubMed Abstruse | CrossRef Full Text | Google Scholar
53. Marsico C, Kimberlin DW. Congenital cytomegalovirus infection: advances and challenges in diagnosis, prevention and treatment. Ital J Pediatr. (2017) 43:38. doi: x.1186/s13052-017-0358-8
PubMed Abstruse | CrossRef Total Text | Google Scholar
54. de Vries JJ, van der Eijk AA, Wolthers KC, Rusman LG, Pas SD, Molenkamp R, et al. Real-time PCR versus viral culture on urine as a gold standard in the diagnosis of congenital cytomegalovirus infection. J Clin Virol. (2012) 53:167–70. doi: 10.1016/j.jcv.2011.11.006
PubMed Abstract | CrossRef Total Text | Google Scholar
55. Boppana SB, Ross SA, Shimamura Yard, Palmer AL, Ahmed A, Michaels MG, et al. Saliva polymerase-concatenation-reaction assay for cytomegalovirus screening in newborns. N Engl J Med. (2011) 364:2111–viii. doi: 10.1056/NEJMoa1006561
PubMed Abstract | CrossRef Full Text | Google Scholar
56. Lanzieri TM, Dollard SC, Josephson CD, Schmid DS, Bialek SR. Breast milk-caused cytomegalovirus infection and affliction in VLBW and premature infants. Pediatrics. (2013) 131:e1937–45. doi: 10.1542/peds.2013-0076
PubMed Abstract | CrossRef Total Text | Google Scholar
57. Wang L, Xu X, Zhang H, Qian J, Zhu J. Dried blood spots PCR assays to screen congenital cytomegalovirus infection: a meta-analysis. Virol J. (2015) 12:lx. doi: 10.1186/s12985-015-0281-ix
PubMed Abstract | CrossRef Full Text | Google Scholar
58. Atkinson C, Emery VC, Griffiths PD. Evolution of a novel single tube nested PCR for enhanced detection of cytomegalovirus DNA from dried claret spots. J Virol Methods. (2014) 196:twoscore–4. doi: ten.1016/j.jviromet.2013.10.029
PubMed Abstract | CrossRef Full Text | Google Scholar
59. Kimberlin DW, Acosta EP, Sanchez PJ, Sood S, Agrawal 5, Homans J, et al. Pharmacokinetic and pharmacodynamic assessment of oral valganciclovir in the treatment of symptomatic congenital cytomegalovirus disease. J Infect Dis. (2008) 197:836–45. doi: 10.1086/528376
PubMed Abstruse | CrossRef Total Text | Google Scholar
60. Luck SE, Emery VC, Atkinson C, Sharland 1000, Griffiths PD. Compartmentalized dynamics of cytomegalovirus replication in treated congenital infection. J Clin Virol. (2016) 82:152–8. doi: 10.1016/j.jcv.2016.07.018
PubMed Abstruse | CrossRef Full Text | Google Scholar
61. Walter Due south, Atkinson C, Sharland Chiliad, Rice P, Raglan E, Emery VC, et al. Congenital cytomegalovirus: association betwixt dried blood spot viral load and hearing loss. Arch Dis Child Fetal Neonatal Ed. (2008) 93:F280–v. doi: x.1136/adc.2007.119230
PubMed Abstract | CrossRef Total Text | Google Scholar
63. Nigro K, Adler SP, La Torre R, Best AM. Passive immunization during pregnancy for congenital cytomegalovirus infection. North Engl J Med. (2005) 353:1350–62. doi: 10.1056/NEJMoa043337
PubMed Abstract | CrossRef Full Text | Google Scholar
64. Blázquez-Gamero D, Galindo Izquierdo A, Del Rosal T, Baquero-Artigao F, Izquierdo Méndez N, Soriano-Ramos M, et al. Prevention and treatment of fetal cytomegalovirus infection with cytomegalovirus hyperimmune globulin: a multicenter report in Madrid. J Matern Fetal Neonatal Med. (2019) 32:617–25. doi: 10.1080/14767058.2017.1387890
PubMed Abstract | CrossRef Total Text | Google Scholar
65. Kagan KO, Enders M, Schampera MS, Baeumel E, Hoopmann M, Geipel A, et al. Prevention of maternal-fetal transmission of cytomegalovirus later on primary maternal infection in the first trimester by biweekly hyperimmunoglobulin assistants. Ultrasound Obstet Gynecol. (2019) 53:383–9. doi: 10.1002/uog.19164
PubMed Abstract | CrossRef Full Text | Google Scholar
66. Revello MG, Lazzarotto T, Guerra B, Spinillo A, Ferrazzi E, Kustermann A, et al. A randomized trial of hyperimmune globulin to prevent congenital cytomegalovirus. Northward Engl J Med. (2014) 370:1316–26. doi: 10.1056/NEJMoa1310214
PubMed Abstract | CrossRef Full Text | Google Scholar
68. Kimberlin DW, Jester PM, Sanchez PJ, Ahmed A, Arav-Boger R, Michaels MG, et al. Valganciclovir for symptomatic congenital cytomegalovirus disease. Northward Engl J Med. (2015) 372:933–43. doi: ten.1056/NEJMoa1404599
PubMed Abstract | CrossRef Full Text | Google Scholar
69. Kimberlin DW, Lin CY, Sanchez PJ, Demmler GJ, Dankner Due west, Shelton Yard, et al. Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the fundamental nervous organization: a randomized, controlled trial. J Pediatr. (2003) 143:16–25. doi: 10.1016/S0022-3476(03)00192-6
PubMed Abstract | CrossRef Full Text | Google Scholar
71. Cho JC, Le Advertizement, Locke SC. Letermovir for prophylaxis of cytomegalovirus in allogeneic hematopoietic stem cell recipients. Drugs Today. (2018) 54:361–8. doi: 10.1358/dot.2018.54.6.2833982
PubMed Abstract | CrossRef Total Text | Google Scholar
72. Le J, Zembillas A, Stanton Thousand, Dahl E, Hanna R, Flagg A, et al. Letermovir for secondary cytomegalovirus (CMV) prophylaxis in a pediatric stalk prison cell transplant patient. Biol Claret Marrow Transplant. (2019) 25:S282–3. doi: 10.1016/j.bbmt.2018.12.354
CrossRef Full Text | Google Scholar
76. Schleiss MR, Permar SR, Plotkin SA. Progress toward evolution of a vaccine confronting congenital cytomegalovirus infection. Clin Vaccine Immunol. (2017) 24:e00268–17. doi: ten.1128/CVI.00268-17
PubMed Abstruse | CrossRef Full Text | Google Scholar
77. Krause PR, Bialek SR, Boppana SB, Griffiths PD, Laughlin CA, Ljungman P, et al. Priorities for CMV vaccine development. Vaccine. (2013) 32:4–ten. doi: 10.1016/j.vaccine.2013.09.042
PubMed Abstract | CrossRef Total Text | Google Scholar
78. N'Diaye DS, Launay O, Picone O, Tsatsaris V, Azria E, Rozenberg F, et al. Toll-effectiveness of vaccination confronting cytomegalovirus (CMV) in boyish girls to prevent infections in pregnant women living in France. Vaccine. (2018) 36:1285–96. doi: 10.1016/j.vaccine.2018.01.042
PubMed Abstract | CrossRef Full Text | Google Scholar
79. Reichman O, Miskin I, Sharoni L, Eldar-Geva T, Goldberg D, Tsafrir A, et al. Preconception screening for cytomegalovirus: an effective preventive approach. Biomed Res Int. (2014) 2014:135416. doi: 10.1155/2014/135416
PubMed Abstract | CrossRef Full Text | Google Scholar
80. de Vries JJ, van Zwet EW, Dekker FW, Kroes AC, Verkerk PH, Vossen AC. The apparent paradox of maternal seropositivity as a risk factor for congenital cytomegalovirus infection: a population-based prediction model. Rev Med Virol. (2013) 23:241–9. doi: x.1002/rmv.1744
PubMed Abstruse | CrossRef Full Text | Google Scholar
schermerhornwilbeend.blogspot.com
Source: https://www.frontiersin.org/articles/10.3389/fped.2020.00013/full
0 Response to "How Many Cmv Babies Are Missed With a Hearing Screen"
Postar um comentário